NOTE – This content is the opinion of laboratory animal science professionals and presented as an educational resource for research programs. Local, national and international guidelines for animal care and use are the primary guidance for all research institutions and the information presented here should never be used in place of those guidelines or in place of consultation with facility leadership responsible for determining care, use and safety standard operating procedures.
Health management definitions
The health management program is a critical component of the global animal care and use program. It includes several components and its complexity depends on the variety of species, the type of research, the animal models used, their health status, etc. One key component is / are the sanitation process(es) required to achieve the objectives.
As a key component of the health management program, sanitation – as defined in the ILAR guide (2011) – includes “the policies, procedures, standards, organizational structure, staffing, facilities, and practices put into place by an institution to achieve an effective, consistent and comprehensive health management of animals, allowing to maintain a predefined animal health status“. A failure in this program may result in poor science, and may generate significant impacts on animal welfare / 3Rs implementation as well as a loss of time, money and reputation.
Adequate equipment and processes, associated to competent staff, are necessary to ensure the suitable levels for bio-exclusion (disease-free, SPF, SOPF, gnotoxenic…) and bio-containment (quarantine, ABSL2 or 3…), used for housing, care and procedures.
A washing area is of often considered the heart of the animal facility, where most sanitation-related flows and processes take place.
At this point, it is worth reviewing a few short definitions and concepts.
- Sanitation is the maintenance of environmental conditions conducive to health and well-being; it involves bedding change, cleaning and disinfection, air quality…
- Cleaning removes excessive amounts of excrement, dirt and debris. Washing is a specific type of cleaning that uses water, through different processes.
- Disinfection reduces or eliminates unacceptable concentrations of microorganisms, mainly pathogenic or undesirable agents (sometimes with the exception of bacterial spores), on inanimate items or surfaces, keeping in mind that only clean items or surfaces can be disinfected.
- Decontamination is the neutralization or removal of dangerous substances to make it safe for (unprotected) personnel.
- Sterilization (required for caging and related supplies in gnotobiology) describes processes that destroy or eliminate all forms of microbial life by physical or chemical methods.
Processes are adapted to the context and the type of research and animal models (especially their health status: conventional / disease-free, SPF, SOPF or gnotoxenic). They may address an objective of bioexclusion, of biocontainment, or both.
Examples of usual processes are:
- In conventional / disease-free unit: adequate washing / rinsing, periodic disinfection → use.
- In ABSL-3 units: decontamination → washing / rinsing → routine disinfection → use.
- In SPF/SOPF units: washing / rinsing → disinfection → use.
- In gnotobiology: washing / rinsing → sterilization → use.
Defining specification for washing area solution
The selecting of washing and disinfection equipment should be based on several criteria, not exclusively the efficacy of the process. Table 1 lists some of these criteria. At the very beginning, a typical “design qualification plan” should start with listing all user requirements and site constraints, then a translation of them into user specifications, then into technical specifications.
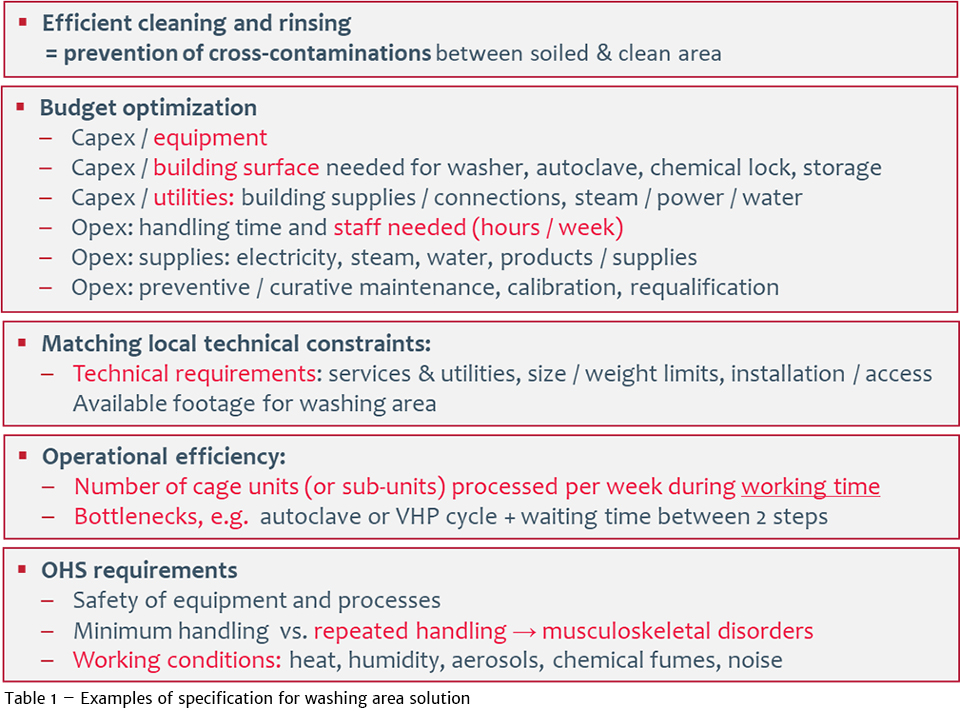
A key rule is to ensure the consistency and global efficacy of the overall health management program, defined according to well-defined objectives. Washing solutions are critical, but are only one of the components that must be aligned to avoid the “weakest link” scenario, which can negatively impact the end-result. Other components include personnel competence, facility deign and flows, good and robust practices, suitable validation…
As shown in Table 1, it is important to stress the importance of identifying all site- constraints: surface and technical limitations or requirements. When selecting technical solutions for washing and disinfection, the objective is to ensure not only the expected level of efficacy for washing, rinsing and disinfection, but also the best operational and OHS / ergonomic performance levels. Additionally, optimized solutions also address equipment, throughput with the absence of bottleneck (e.g. due to repetitive handling for loading / unloading, loss of time, insufficient equipment capacity), using the minimum footprint, costs, and environmental impact… As an example, consumption of water, energy (electrical power, steam production), detergent use… should be minimized.
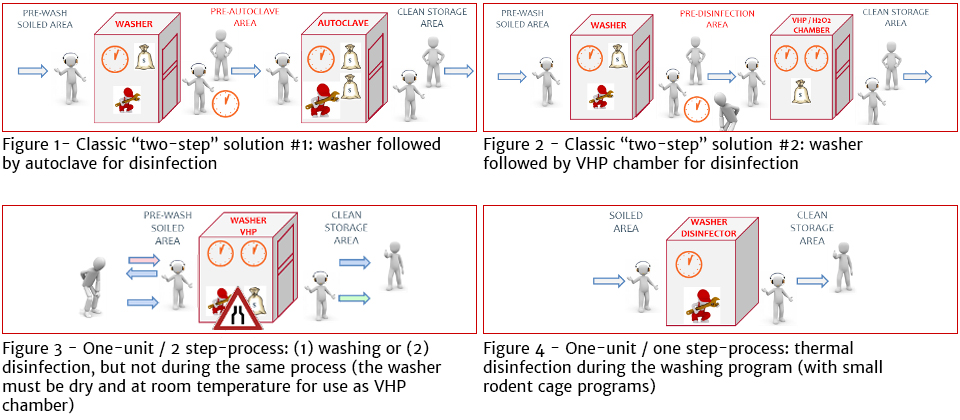
Figures 1 to 4 illustrate some integrated solutions combining washing and disinfection.
Thermal disinfection solutions
One particular issue is to define the need for an autoclave, which is the most expensive equipment to buy, maintain and operate. Things to consider are its design, capacity, function (sterilization or biohazard decontamination use), type of programs required, etc.
In animal units, an autoclave is required for gnotobiology (sterilization of caging and / or supplies) or for biocontainment (wasted / soiled equipment decontamination) and for thermal disinfection of porous loads (bedding, feed, disposable enrichment items…) when alternatives are not available (such as gamma radiation for feed and bedding or reverse osmosis for water).
Washer-disinfectors have been used in hospitals for many years, but currently are barely considered as a solution in research animal facilities. The thermal disinfection is a part of the washing program and is based on the A0 concept, i.e. the time of exposure of a load at 80°C. In hospitals, a A0=60 (80°C during a plateau of 60 sec., or a higher temperature during a shorter time) is the value applicable to equipment heavily soiled by feces, to be re-used in contact of normal skin of patients, including those sensitive to infections. In small rodent facilities, it could be applicable to caging components from barrier units housing SPF or SOPF rodents. In hospitals, a A0 = 600 value (80°C during a plateau of 600 sec., or a higher temperature during a shorter time) is applicable to medical devices (such as endoscopes) between patients. In rodent facilities, it could be applicable to special / more demanding situations, e.g. during a contamination.
A washer-disinfector is a washer that permits a thermal disinfection step during the washing process. In rack washers, it is managed by steam injection in the chamber at the end of the process (after rinsing). In a cabinet washer, it is conducted at high-temperature during pre-rinsing, using one tank with a suitable heating capacity.
Both allow using less expensive equipment and operational costs, with a shorter time and an increased sanitation capacity. As with other thermal disinfection processes, it can be validated and controlled (time of “plateau” thermal exposure).
With cabinet washers, this technical solution is usable with small rodent cages, when one of the two recirculation / heating tanks is used for washing and the other one is available for thermal disinfection during pre-rinsing. Rinsing is the last step, with non-recirculating water at high temperature, i.e. during a shorter duration.
Due to the capacity of the chamber and / or budget constraints related to technical solution (pumps), the water temperature during pre-rinsing in cabinet washers is generally limited to about 82°C, which is already compatible with an excellent performance level.
Validation / performance qualification and indicators
Before the acquisition of a technical solution, the design qualification (DQ) demonstrates that the selected design satisfies or will satisfy all the requirements that are defined and detailed in the User Requirements / Specification (URS) and that the design can be authorized. After delivery, the installation qualification (IQ) process demonstrates that the process or equipment meets all specifications, is installed correctly, and all required components needed for continued operation are installed and in place. Then, the operational qualification (OQ) demonstrates that all facets of the process or equipment are operating consistently. Finally, it is the responsibility of the end user to conduct the performance qualification (PQ) to verify and document the performance of the equipment in order to assess whether or not the expected performance is achieved. These steps are mandatory in GMP facilities or in ABSL-3/4 units, but are not always conducted in other animal facilities.
The expected result of disinfection (pro sterilization, or decontamination) must be reached at any level / spot of each defined load used with the equipment (washer/disinfector, autoclave or chemical chamber) using a suitable number of relevant indicators (temperature, chemical, physical, microbiological indicators, adapted to the nature of each process). It is common to use at least two steps, with two different indicators. As an example, for thermal disinfection or sterilization, temperature probes (such as data loggers) are used as a first step, followed by the use of biological indicators adapted to the technology used.
Recently, I presented a webinar with my colleague Kevin Nederfield that addressed the challenges and choices facing research institutions seeking to select washing and contamination control systems that fit their space, their need, and their budget. To view this webinar on-demand, click here.

