The purpose of the Sentinel EAD (Exhaust Air Dust) is to provide researchers with the most comprehensive, sensitive and specific method for rodent colony health assessment.
It is now widely accepted that soiled-bedding sentinel programs are not completely reliable, as many agents do not transfer well to sentinel animals. Plenum and cage swabbing helps increase the accuracy of heath screening over soiled bedding sentinel programs, but is neither as labor-efficient nor accurate as Sentinel EAD sampling associated to qPCR testing.
As illustrated in FIGURE 1, the Sentinel EAD system relies on the airflow features both at cage and at rack exhaust level, the position of the capture system, the design of the holder, the nature of the capture media and, last but not least on the sensitivity and specificity of qPCR technology currently available. Particles generated by the animals at cage level are immediately captured by the cage airflow and kept suspended within the airflow until they reach our specialized capture media placed at rack exhaust level. This ensures relevant samples from currently housed animals.
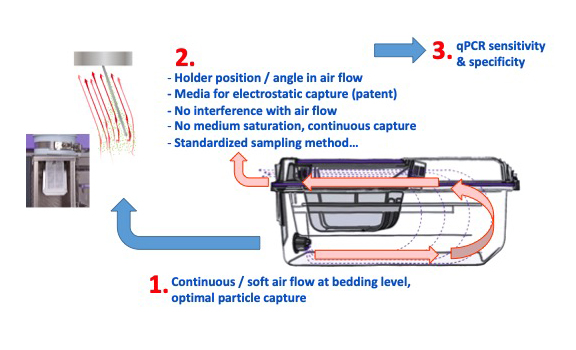
Thanks to all of its features, the Sentinel EAD system reduces the risk of false negative and false positive results.
Indeed, while concerns over residual nucleic acid primarily arise with the use of swabbing method in combination with PCR, the Sentinel EAD system may not suffer from that drawback, as it does not require ‘‘digging’’ in soiled bedding or dusty plenums with a swab. The likelihood that residual material would be re- aerosolized from plenum dust and captured in sufficient amount on the Sentinel EAD media is very low.
In addition to allowing quantification, qPCR is also more specific than conventional PCR, thanks to the additional specificity provided the probe sequence that includes the fluorescence reporter and the quencher. This is allowing a better control of the risk of false positive.
TABLE 1 lists the agents for which the capture by Sentinel EAD could be demonstrated during real condition use. All types of agents could be detected (viruses, bacteria, endoparasites and ectoparasites). It should be emphasized that when an agent was not detected by Sentinel EAD, it was not detected either by other methods. In some cases, agents known as present historically in a colony may not be detected by any method during one screening time: it is generally assumed that this may be due to periodic shedding of the agent, a common feature of opportunistic agents. In this case, a continuous capture over several weeks is obviously the best way to decrease the risk of shedding-dependent results.
TABLE 1 – Agents detected by Sentinel EAD
“Environmental Mouse Surveillance Plus” screening profile (CRL)
Test results in real conditions, Europe & USA
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| x = capture demonstrated | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
All agents “non captured” by the “in line system” were not detected by any other method.
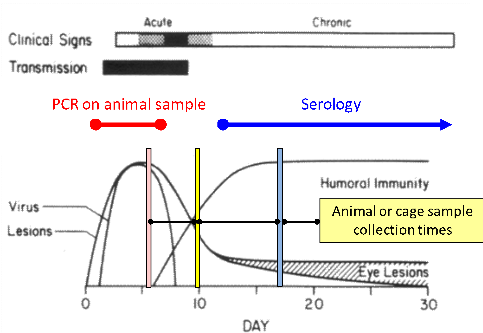
It is worth mentioning that particle capture is permanent, i.e. over the whole period of use of the Sentinel EAD media. As shown in FIGURE 2, even if the duration of shedding of an agent is limited, it will be collected and kept on the media until testing. This is a major advantage over direct animal sampling for PCR testing, which can lead to false negative if the sample is collected after the shedding period (e.g. of MHV or RCV).